As part of our training program, we regularly challenge our technicians to show us how they have progressed in their training.
We asked David, our new lab technician to prep a metallurgical sample to a final polish and then etch it to show how heat treatments affect the grain structure of a metal.
For thousands of years, metal has been heat-treated to extract performance, primarily to make it stronger. Metals are heated, shaped, and cooled repeatedly to produce the desired outcome.
In this case, we found an open-ended wrench on the ground at a local automotive junkyard (junkyards are great sources of metallurgical samples to play with). We cut the top off the wrench and David went to work.
What happens when the metal is heat-treated?
We assumed the wrench would be hardened and we would see the difference in grain structure once we etched the sample. The change on the grains would show how deep the heat treatment penetrated and what kind of change had happened.
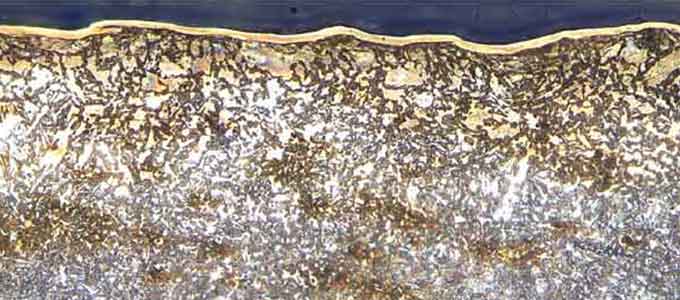
In the image below, taken with a 5X objective, you can clearly see how far the desired heat treatment penetrated the wrench. The top thin layer is for corrosion resistance (we can assume chrome). The shading from dark to light changes as the grain structure changes. The rust-looking spots are just that, corrosion that happened as we let the polished, unprotected, wrench sit in our lab.

In the higher magnification image below, taken with a 50X objective, you can clearly see the metal grains are of a larger size closer to the surface of the wrench.
This is where the grains are most affected by the heat treatment. The grains get smaller the deeper they go.
For information, please visit our Analytical Lab Services page at the link below, or complete the form on this page to contact our lab staff.




